Are you looking for an answer to the topic “Are hypertonic and Hyperosmotic the same?“? We answer all your questions at the website Chambazone.com in category: Blog sharing the story of making money online. You will find the answer right below.
Hyperosmotic solutions are not always hypertonic. But hyposmotic solutions are always hypotonic. The response to this rapid fire presentation of osmolarity and tonicity was overwhelmingly positive.As adjectives the difference between hypertonic and hyperosmotic. is that hypertonic is (of a solution) having a greater osmotic pressure than another while hyperosmotic is hypertonic.The key difference between isosmotic hyperosmotic and hypoosmotic is that isosmotic refers to the property of having equal osmotic pressures, but hyperosmotic refers to the property of having a high osmotic pressure. Meanwhile, hypoosmotic refers to the property of having a low osmotic pressure.
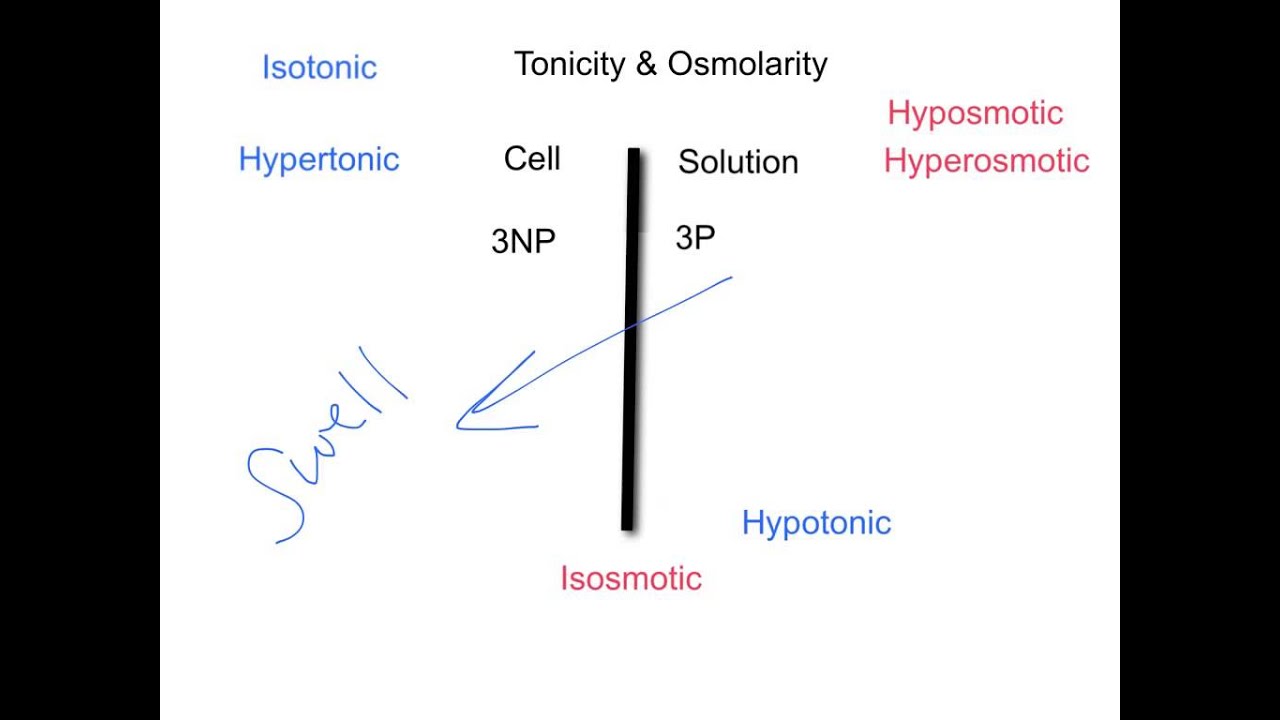
What is the difference between hypertonic and hyperosmolar?
As adjectives the difference between hypertonic and hyperosmotic. is that hypertonic is (of a solution) having a greater osmotic pressure than another while hyperosmotic is hypertonic.
What is the difference between Hypoosmotic and Hyperosmotic?
The key difference between isosmotic hyperosmotic and hypoosmotic is that isosmotic refers to the property of having equal osmotic pressures, but hyperosmotic refers to the property of having a high osmotic pressure. Meanwhile, hypoosmotic refers to the property of having a low osmotic pressure.
Tonicity Osmolarity
Images related to the topicTonicity Osmolarity

What does it mean if a solution is Hyperosmotic?
Hyperosmotic (biology definition): (1) of, relating to, or characterized by an increased osmotic pressure (typically higher than the physiological level); (2) a condition in which the total amount of solutes (both permeable and impermeable) in a solution is greater than that of another solution.
What is the difference between osmolality and tonicity?
Osmolality is a property of a particular solution and is independent of any membrane. Tonicity is a property of a solution in reference to a particular membrane.
Does Hyperosmotic mean hypotonic?
A 5 percent dextrose solution with no non-penetrating solutes is a classic example of a hypotonic solution. Water movement occurs when a cell is put in a hyperosmotic yet hypotonic solution, such as 10% dextran. As a result, a solution might be hyperosmotic and hypotonic at the same time.
Are osmolarity and osmolality the same?
Osmolarity and osmolality are frequently confused and incorrectly interchanged. Osmolarity refers to the number of solute particles per 1 L of solvent, whereas osmolality is the number of solute particles in 1 kg of solvent.
Is Hypoosmotic and hypotonic the same?
Hyperosmotic solutions are not always hypertonic. But hyposmotic solutions are always hypotonic. The response to this rapid fire presentation of osmolarity and tonicity was overwhelmingly positive.
See some more details on the topic Are hypertonic and Hyperosmotic the same? here:
Tonicity: hypertonic, isotonic & hypotonic solutions (article)
If a cell is placed in a hypertonic solution, water will leave the cell, and the cell will shrink. In an isotonic environment, there is no net water movement, …
Hyperosmotic Definition and Examples – Biology Online
What is hyperosmotic? The word hyperosmotic is derived from two Greek words: ‘hyper’, meaning “excess” and ‘osmos’, meaning “thrust” or “push …
Question about the difference of Hyperosmolar vs Hypertonic?
Hyperosmolar and Hypertonic seem to be the same? They both have the higher concentration, or more solutes. As if you where to drop a cell …
Hypertonic vs Hyperosmotic – What’s the difference? | WikiDiff
As adjectives the difference between hypertonic and hyperosmotic. is that hypertonic is (of a solution) having a greater osmotic pressure than another while …
Is hypertonic low to high concentration?
Hypertonic: The solution with the higher concentration of solutes. Hypotonic: The solution with the lower concentration of solutes.
Can a solution be Hyperosmotic and isotonic?
Non-penetrating solutes cannot cross the cell membrane; therefore, the movement of water across the cell membrane (i.e., osmosis) must occur for the solutions to reach equilibrium. A solution can be both hyperosmotic and isotonic.
What is a Hyperosmotic fluid?
Hyperosmotic can refer to solutions that have increased osmotic pressure, or a greater difference between solutes and solutions between a membrane. In other instances, hyperosmotic refers to a solution that has more solutes, or components of a solution, than a similar solution.
What happens to a cell in a Hypoosmotic solution?
Under these conditions, the osmotic pressure gradient forces water into the cell. Depending on the amount of water that enters, the cell may look enlarged or bloated. If the water continues to move into the cell, it can stretch the cell membrane to the point the cell bursts (lyses) and dies.
What is Isoosmotic?
iso-osmotic (not comparable) Having the same solute concentration, and therefore the same water potential; frequently construed with with.
LPA 1B – Osmolarity vs Tonicity
Images related to the topicLPA 1B – Osmolarity vs Tonicity

Is high osmolarity hypertonic?
In a hypertonic solution, the extracellular fluid has a higher osmolarity than the fluid inside the cell; water leaves the cell.
What is the relationship between osmosis osmolarity and tonicity?
The ability of an extracellular solution to make water move into or out of a cell by osmosis is known as its tonicity. Tonicity is a bit different from osmolarity because it takes into account both relative solute concentrations and the cell membrane’s permeability to those solutes.
What is the difference between osmosis and tonicity?
Osmosis describes the number of solutes dissolved in a volume of solution. It has units whereas tonicity has no units. Osmolarity is comparing two solutions. Tonicity is comparing a solution and a cell.
Does hypertonic shrink or swell?
A hypotonic solution causes a cell to swell, whereas a hypertonic solution causes a cell to shrink.
Is 0.9% NaCl hypertonic or hypotonic?
Hypertonic Solution
If a cell with a NaCl concentration of 0.9% is placed in a solution of water with a 10% concentration of NaCl, the solution is said to be hypertonic.
What will happen if a normal cell is placed in a Hyperosmotic solution?
What will happen if a normal cell is placed in a hyperosmotic solution? It may swell, shrink, or stay the same size, depending upon the concentration of penetrating and nonpenetrating solutes in the solution.
How do you remember the difference between osmolarity and osmolality?
The difference is subtle between osmolarity and osmolality. I mean there is only one letter different in the words. Osmolality is measuring the number of osmoles in a weight (kg) of solvent. Osmolarity is measure the number of osmoles in a volume (L) of solvent.
Is osmotic pressure the same as osmolality?
Osmolality (or osmolarity) should be used instead of osmotic pressure to describe the movement of water between compartments while the use of osmotic pressure should be reserved for situations where filtration and osmosis are operating together.
What is in hypertonic solution?
Hypertonic solution: A solution that contains more dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood.
What is the difference between hypertonic hypotonic and isotonic?
Hypotonic has a lower concentration of fluid, sugars and salt than blood. Hypertonic has a higher concentration of fluid, sugars and salt than blood. Isotonic has similar concentration of fluid, sugars and salt to blood.
Hypertonic, Hypotonic and Isotonic Solutions!
Images related to the topicHypertonic, Hypotonic and Isotonic Solutions!

What causes Hyperosmotic stress?
An imbalance between extracellular and intracellular fluid osmolarity, and therefore osmotic pressure, is the underlying cause of osmotic stress. By definition, when extracellular fluid osmolarity is greater than that of the intracellular fluid, cells and tissues experience hyperosmotic stress.
Is normal saline hyperosmolar?
0.9% saline is a perfectly isotonic solution that is isoosmolar to human plasma and is not associated with hypernatremia [2].
Related searches to Are hypertonic and Hyperosmotic the same?
- isosmotic solution example
- is hypertonic or hypotonic more dangerous
- hypotonic
- isotonic, hypertonic hypotonic
- what patients get hypertonic solutions
- does a hypertonic solution cause a cell to swell
- why are hyperosmotic solutions always hypotonic
- tonicity definition biology
- isotonic hypertonic hypotonic
- tonicity vs osmolarity
- isosmotic vs isotonic
- hypotonic solution
- hypertonic vs hypotonic
- what is the difference between hypertonic and hyperosmotic
- are hypertonic and hyperosmotic the same
- can a hyperosmotic solution be hypotonic
- is saliva hypertonic or hypotonic
Information related to the topic Are hypertonic and Hyperosmotic the same?
Here are the search results of the thread Are hypertonic and Hyperosmotic the same? from Bing. You can read more if you want.
You have just come across an article on the topic Are hypertonic and Hyperosmotic the same?. If you found this article useful, please share it. Thank you very much.
